Use of charcoal as fuel
In economically developed countries, charcoal is widely used for lighting barbecues when preparing barbecues, shish kebab, etc.
Elsewhere in the world, charcoal is used daily for cooking.
This is a serious threat to human health, since food preparation does not take place outdoors, but indoors. Respiratory infections caused by burning charcoal indoors are the cause of death among children under 5 years of age in all third world countries. That's almost 2 million deaths a year. Carbon monoxide also pollutes the atmosphere.
How to use it correctly
Since fertilizer has the property of reducing soil acidity, before using it you need to determine the composition of the soil in the garden plot. If the soil in the garden contains a large amount of alkali, you should discard charcoal. The fertilizer is suitable for neutral soil. Burnt wood helps make the soil looser and softer. Seeds germinate much better on such soil. Therefore, ash is also used before planting, not only as a top dressing. The ability of burnt wood to improve gas exchange stimulates the growth of microorganisms.
Fertilizer is applied to the soil in a special way. The substance is diluted with water in a proportion of up to 3 glasses per square meter. It’s easy to use charcoal undiluted: you just need to sprinkle it around a plant or shrub. In the future, together with precipitation, it will descend deep into the ground. The ash is also used for seedlings. To do this, pour a layer of 2-3 cm into the tray of the box and cover it with soil on top. This results in a kind of drainage that constantly provides the plants with nutrition. It is better to use charcoal in May and early June, when crops are just beginning to grow. This will allow them to bloom, develop and bear fruit faster.
Crushed coal is a universal fertilizer that can be used for various crops. As a result, productivity increases significantly, people use an environmentally friendly product, without harm to the environment and health.
The use of charcoal as automobile fuel
During times of shortage of petroleum products, cars were redesigned to run on mixed gas - a gas mixture containing mainly carbon monoxide CO. Mixed gas was produced by burning coal or wood in gas generators. In China, for example, until 1950, cars that used coal as fuel were popular. During World War II, European production of wood and charcoal as fuel for automobiles increased approximately 10-fold.
Charcoal
Details Category:
CHARCOAL
, a product obtained from wood by heating it to high temperatures (350-600°) without or with little access to air. The greater the access of air during charcoaling, the lower the yield of coal from wood, the looser and lighter the coal itself. Real black charcoal, completely suitable for consumption, is obtained at temperatures of 350° and above. The carbon content in charcoal does not depend on the type of wood, but solely on the temperature of re-charring. The effect of temperature on the decomposition of wood and the chemical composition of the resulting coal is presented in Table. 1, according to the latest data by V. A. Korobkin.
From this it is clear that even at 1500° it is impossible to completely free coal from the gas components of wood - hydrogen and oxygen, while in ordinary coal their content is slightly less than 25%. From this table it follows that raising the charcoal temperature above 600° is irrational, since subsequent intervals of 100° slightly increase the content of valuable carbon. The volumetric specific gravity varies significantly depending on the method, temperature and firing rate, averaging for coal from Schwartz kilns in the Nizhny Tagil district in the Urals: spruce 0.235, pine 0.267, aspen 0.289 and birch 0.365 with fluctuations up to 50%.
The true specific gravity, determined on fine crushed coal, gives values of about 1.3-1.4 for the same coals. The calculated porosity of coal was 70–80%. In table Figure 2 shows the relationship between the carbon content, porosity and specific gravity of coal from different forest species.
In direct contact with water, coal absorbs it from 200 to 300%, calculated by the weight of coal dried at 105°. The average weight of stale coal from Schwartz furnaces is: spruce 120 kg, pine 140 kg, aspen 150 kg per stacked m3. The mechanical compressive strength of coal (from Schwartz furnaces) is approximately four times less than the original strength of the corresponding wood. The calorific value of charcoal is twice that of the wood from which it is obtained, and for good grades exceeds 7000 Cal. The pyrometric effect of burning coal is also significantly higher than wood. In table Table 3 shows the chemical composition and physical properties of stove coal.
The thermal properties of charcoal and its chemical composition (the absence of impurities that harm the quality of the product, the low content of non-caking ash that does not require flux) are used in blast furnaces for smelting cast iron from iron ores, for cupola furnaces for iron casting, and for the production of cement steel. , for forges for hot working and welding of iron. The best coal for blast furnace smelting is considered to be smetnichny coal - a mixture of birch, pine and spruce coals. Good charcoal is distinguished by its shiny black color, density, makes a ringing sound when falling, burns without flame or smoke and does not stain your hands.
According to the method of production, they distinguish: 1) pit charcoal, 2) campfire, or heap, 3) stove and 4) retort, or cauldron. These coals differ significantly from each other in their chemical, physical and mechanical properties.
Yamny method
. A round hole with a diameter of about 2 m and a depth of 1.5 m is dug in dry soil. Small dry branches are thrown to the bottom and a fire is lit. When the branches flare up, charred material is piled on them and, gradually adding it, the entire pit is filled with burning wood. After this, turf and soil are thrown on top, the tire is compacted by tamping and the pit is left closed. After a day, you can already rake out the finished coal. The remaining pockets of smoldering coal are poured with water during excavation. The coal is fine, friable, and has a low specific gravity. Yields by weight are 10-12%, and by volume 30-35%. Such coal is used for village forges and for household needs. Handicraft charcoal burners in the southern parts of the RSFSR use only branches and tree tops for the pit method. In the Leningrad region and in the Central Industrial Region, firewood is used for charcoaling, and here the pit method is modified: the firewood is laid in dense rows in an oblong pit, covered with branches and turf, and after that it is lit through the hole left in the tire. At the end of the process, the hole is filled up, the surface of the pit is compacted and, when the coal has cooled, it is raked out. This method improves the quality of coal and increases the yield.
Campfire method
Charcoalization produces industrial metallurgical coal of the best quality. However, in the Urals, only 5% of fire charcoal is currently burned, while in Sweden, where the consumption of charcoal by metallurgy is slightly inferior to the Urals, still more than two-thirds of all coal is produced by the fire method. Fires, or piles, can be standing, with a vertical position of firewood, or lying, with a horizontal position. The most common and more advanced are standing fires. In the Urals, the type of fire shown in the section in Fig. is very common. 1. In a specially selected dry area, the current is leveled and a tall stake is driven into its center. If the place is damp, then it is necessary to dig a ditch over the place of the fire, and under the fire itself to arrange a flooring of chopped firewood - bridge (a) (Fig. 1 and 2), in order to avoid getting a large amount of poorly charred hoof grass.
Firewood is placed to one side of the stake so that an incendiary channel is formed (b). Firewood for the fire is taken dry, one-year-old, 1 m long. The uppermost tier (c) is called a cap, but sometimes it is not made, laying the firewood of the third tier with a large slope towards the center of the fire. After laying the firewood, the surface of the fire is covered with pine branches and an earthen cover is also placed on them. The tire layer is thinner at the top than on the sides. The success of charcoaling depends on the composition of the tire and the best is considered to be loamy soil mixed with ash and fine coal remaining from the previous charcoaling.
Clay soil cracks, while sandy soil crumbles from the sides when it dries. Typically, a Ural fire holds 100-120 m3 of wood. The fire is ignited through channel (b). At first, moisture is released abundantly from the firewood, which partially settles on the tire (sweating from the fire). If the wood is drying slowly, the charcoal burner removes part of the earthen cover from the cap, and below the bridge he punches holes on the leeward side (cellars, or thresholds) to increase air access. When the yellow smoke gives way to transparent, bluish smoke and the top of the fire begins to settle, the process of charcoaling at the top of the fire is completed; The coal burner increases the layer of cover here, trying to stop the access of air to the finished coal. The fire is transferred to the second tier of firewood, reducing the layer of tire here or punching a series of small holes in it. Here, too, yellow smoke is first produced, which gradually turns blue, and then the fire is transferred to the lower tier, regulating the progress of charcoal burning in the last stage by opening or filling the basements. In the first stage of the process, explosive mixtures of gases can form in different parts of the fire, and the fire “shoots,” throwing off a tire in places and sometimes throwing out firewood. If the fire is laid incorrectly or due to lack of supervision, the coal burns out in some places, resulting in failures. In all such cases, the charcoal burner must immediately fill the resulting voids with fresh firewood (feeding the fire) and completely restore the original layer of the tire. At the end of the firing, all the basements are filled up, the heap is allowed to cool, which takes 1-2 days, and they begin to dismantle the heap, starting with the cap. Disassembly is carried out only in dry weather, so as not to stain the finished coal. When the coal has completely cooled, fines (patya) are sifted out on a screen and the coal is placed either in matting coolers or in boxes woven from twigs for transportation to iron smelters. The box in the Urals serves as an accounting unit, containing 2 m3 of coal. Charcoaling continues, depending on the weather, for 8-10 days, and the same amount of time is spent on building a pile, breaking and cleaning the coal. In total, a fire of 120 m3 requires up to 50 man-days.
In Western Europe, standing fires are arranged a little differently (Fig. 3). The current is made on an artificial hill with a rise towards the center, and the bridge (a) is arranged in any kind of ground.
The incendiary channel (b) is made vertical from four stakes driven at some distance. A significant amount of incendiary material (c) is piled up in the center of the fire in order to immediately set off energetic charcoaling. The cap (d) is laid out with special care, since its correct shape contributes to the normal course of the process. The capacity of such a fire is twice that of the Ural fire (250 m3). Charcoaling is, in general, similar to the Ural method, but, due to the peculiarities of the fire structure, the process of coal formation spreads along a paraboloid (Fig. 4).
Supervision of the progress of the “German” fire is carried out more carefully, and feeding of the fire is especially often practiced. The coal yield in Ural standing fires is 20-22% by weight and 50-55% by volume of air-dried one-year-old firewood; the yield in German bonfires is higher, amounting to 24% by weight and 60-65% by volume. Coniferous species, especially spruce, give higher yields; birch gives about 18% by weight and 45% by volume. The charcoaling temperature in fires is 500-600°, and according to this, fire coal contains about 90% carbon in elemental composition, with the amount of non-volatile carbon being 82-84%. The last figure is especially important for the blast furnace process, since it is the content of non-volatile carbon that determines the quantitative yield of cast iron, while volatile carbon helps to increase the yield and improve the composition of only the blast furnace gases. If these latter are not used, then the yield of cast iron is the only criterion for assessing the quality of coal, and in this regard, camp coal has the best performance. The volumetric specific gravity of campfire charcoal and its mechanical properties are higher than those of other types of charcoal.
Recumbent fires (Fig. 5) are arranged between stakes driven into the edges. The width of the fire is slightly longer than the length of the logs, and the length may be. arbitrary (3-5 times the width); height is about 2 m. Boards are leaned against the stakes and, at the same time as the logs are laid, an earthen backfill is made between the boards and the ends of the logs. At one end of the fire, the wood is stacked higher to create a direction of draft. Lighting the fire begins from the low edge (a), which is called the sole, and directs the fire to the high edge (b) - the sail, by punching holes in the top cover of the fire. When conducting the process, they are guided by the same signs as in standing fires. Coal yields are slightly less than in standing fires, but the coal itself turns out to be more crushed, since its strength in the direction perpendicular to the fibers is approximately 5 times less compared to the strength in the direction parallel to the fibers, and with a horizontal arrangement of logs a lot of finished coal is crushed in the lower rows.
The difficulty of controlling the progress of the process during charcoaling, dependence on the weather, the shortness of the charcoaling season, the need to build a fire cover each time, maintain it in good condition during charring and disassemble it at the end prompted technicians to introduce charcoal kilns. An example of such a furnace is the Schwartz furnace (Fig. 6), which is a rectangular oblong chamber with a firebox located under the middle of the longitudinal wall.
The firebox without a grate is located under the brick hearth of the furnace, and the combustion gases enter the charcoal chamber through an opening in the center of the hearth. Opposite the firebox in the other longitudinal wall there is a loading hole, instead of which, to speed up loading, sometimes two holes are installed in the transverse walls of the furnace. There are 4 wooden exhaust pipes in the corners of the stove. The stove is covered with a light roof on wooden pillars, which also serve to connect the stove. Having densely loaded the stove with firewood, located vertically at the bottom (standing), and horizontally under the arch (stopper), close the loading hole with an iron flap and coat the seams with clay. A fire is lit in the firebox, and hot flue gases, penetrating into the furnace, gradually increase the temperature of the charcoal burning chamber. First, white smoke flows through the exhaust pipes, then yellow, and the appearance of bluish transparent smoke serves as a sign of the end of the process, after which the combustion and exhaust openings are tightly closed, and the stove cools down for several days. After unloading the coal, the furnace is ready for new loading. With a stove capacity of 50 m3 of wood, it makes 3.5 revolutions per month; a furnace of 70 m3 makes 3 revolutions and a furnace of 100-120 m3 makes 2.5 revolutions. Productivity is higher for small-volume furnaces, but maintenance and fuel consumption for large-volume furnaces give more economical results, which is why, on average, furnaces of 60-80 m3 are the most profitable. For fuel, you can use the worst firewood, twigs, chips, etc. Fuel consumption is 6-10% of the volume of loaded firewood, and coal yields, including fuel firewood, reach 88% by volume and 26% by volume in Schwartz furnaces for spruce weight, for pine, respectively - 80 and 25% and for birch - 58 and 23%. The volumetric output is taken into account in boxes, in which, however, there are many voids between the pieces of coal. The total carbon content is 76-80% with 67% non-volatile carbon. Such a low carbon content in coal is entirely determined, as Yuon first pointed out, by the low temperature in Schwartz furnaces, amounting to only 350-400°, as opposed to 500-600° achieved in fires.
To increase the firing temperature in kilns and improve the quality of coal, they resort to gas fuel, which has a higher pyrometric effect, using gas workers directing gaseous decomposition products into the furnace, or arranging gas generator furnaces. F.N. Sukhanov improved the Schwartz firebox and introduced a device for blowing air, due to which the gaseous products of wood decomposition burn at the site of their formation and thereby greatly increase the temperature of the process; the temperature under the roof in these furnaces exceeds 700°, the quality of coal produced is not lower than that of good campfire coal, but the volumetric and weight yields are significantly lower than in Schwartz furnaces.
The same principle of utilization of the resulting combustible gases for heating wood is implemented in retort furnaces (see Wood, dry distillation).
To increase the mass yield of coal and reduce its cost, continuous furnaces with utilization of liquid products are being introduced. In the Urals, the cost of coal from continuous furnaces exceeded twice the cost of coal from Schwartz furnaces, and the disposal of alcohol and acetic acid would be profitable only with birch firewood. Until 1914, the Urals consumed almost 12 million m3 of wood for burning charcoal, and in 1928/29 the consumption of metallurgical coal will already be about 85% of this amount. If we consider that the dry distillation of deciduous wood throughout the RSFSR consumes only 400,000 m3 of wood, then the full importance of charcoal in the national economy of the Union becomes clear. When processed, charcoal cast iron produces special grades of iron and steel, which are significantly superior in quality to those made from coke cast iron.
Source: Martens. Technical encyclopedia. Volume 7 - 1929
- < Back
- Forward >
The use of charcoal in cleaning and filtration equipment
Activated carbon has high adsorption due to the large number of pores and large specific surface area per unit mass. Activated carbon quickly adsorbs a wide range of organic compounds dissolved in gases or liquids. Charcoal is often used to filter water to remove harmful bacteria. In the East, in countries such as China, Japan, Korea, water purified with charcoal or bamboo is used to make tea.
In some industrial applications, such as sugar refining from sugarcane, impurities that cause undesirable colors can be removed using activated carbon.
Activated carbon is used to absorb odors and toxins from the air. Filters made from charcoal are also used in some types of gas masks.
The use of activated carbon in medicine consists mainly in the adsorption of poisons entering the human body. It is also used to reduce discomfort in the digestive tract.
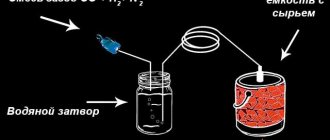
Charcoal production equipment
You can make fuel at home using improvised means and even in an earthen pit. For industrial production, retort-type combustion furnaces are used. These can be either stationary or mobile installations. The main element of such installations is the combustion container. The pyrolysis barrel is the most expensive element of the installation. Today, manufacturers are offered several options for industrial installations, which include a full set of equipment for the job.
What is coal made of? What is the chemical formula of coal
Coal is one of the oldest fuels known to man. And even today it occupies a leading position in terms of volume of use. The reason for this is its prevalence, ease of extraction, processing and use. But what is he? What is the chemical formula of coal?
In fact, this question is not entirely correct. Coal is not a substance, it is a mixture of different substances. There are a lot of them, so it is impossible to completely determine the composition of coal. Therefore, by the chemical formula of coal in this article we will rather mean its elemental composition and some other features.
But what can we learn about the state of this substance? Coal is formed from plant remains over many years due to exposure to high temperature and pressure. And since plants are organic in nature, organic substances will predominate in the composition of coal.
Depending on the age and other conditions of origin of coal, it is divided into several types. Each type differs in its elementary composition, the presence of impurities and other important characteristics.
Brown coal
It is the youngest type of coal. It even exhibits a plant-like woody structure. It is formed directly from peat at a depth of about 1 kilometer.
This type of coal contains a fairly large amount of moisture: from 20 to 40%. When exposed to air, it evaporates and the coal crumbles into powder. Next we will talk about the chemical composition of this particular dry residue. The amount of inorganic impurities in brown coal is also large and amounts to 20-45%. These impurities include silicon dioxide, aluminum, calcium and iron oxides. It may also contain alkali metal oxides.
This coal contains a lot of volatile organic and inorganic substances. They can make up up to half the mass of this type of coal. The elemental composition excluding inorganic and volatile substances is as follows:
- Carbon 50-75%.
- Oxygen 26-37%.
- Hydrogen 3-5%.
- Nitrogen 0-2%.
- Sulfur 0.5-3%.
Coal
In terms of formation time, this type of coal comes next after brown coal. It has a black or gray-black color, as well as a resinous, sometimes metallic sheen.
The moisture content of hard coal is significantly less than brown coal: only 1-12%. The content of volatile substances in coal varies greatly depending on the location of mining. It can be minimal (from 2%), but can reach values similar to brown coal (up to 48%). The elemental composition is as follows:
- Carbon 75-92%.
- Hydrogen 2.5-5.7%.
- Oxygen 1.5-15%.
- Nitrogen up to 2.7%.
- Sulfur 0-4%.
From this we can conclude that the chemical formula of hard coal consists of more carbon than that of brown coal. This makes this type of coal a higher quality fuel.
Anthracite
Anthracite is the oldest form of fossil coal. It is characterized by a dark black color and has a characteristic metallic sheen. This is the best coal in terms of the amount of heat it releases during combustion.
The amount of moisture and volatile substances in it is very small. About 5-7% for each indicator. And the elemental composition is characterized by an extremely high carbon content:
- Carbon over 90%.
- Hydrogen 1-3%.
- Oxygen 1-1.5%.
- Nitrogen 1-1.5%.
- Sulfur up to 0.8%.
More coal is contained only in graphite, which is a further stage of anthracite coalification.
Charcoal
This type of coal is not a fossil, so it has some peculiarities in its composition. It is produced by heating dry wood to a temperature of 450-500 oC without air access. This process is called pyrolysis. During this process, a number of substances are released from the wood: methanol, acetone, acetic acid and others, after which it turns into coal. By the way, burning wood is also pyrolysis, but due to the presence of oxygen in the air, the released gases ignite. This is what determines the presence of flames during combustion.
Wood is not homogeneous; it has a lot of pores and capillaries. A similar structure is partially preserved in the coal obtained from it. For this reason, it has good adsorption capacity and is used along with activated carbon.
The humidity of this type of coal is very small (about 3%), but during long-term storage it absorbs moisture from the air and the percentage of water increases to 7-15%. The content of inorganic impurities and volatile substances is regulated by GOSTs and should be no more than 3% and 20%, respectively. The elemental composition depends on the production technology, and looks approximately like this:
- Carbon 80-92%.
- Oxygen 5-15%.
- Hydrogen 4-5%.
- Nitrogen ~0%.
- Sulfur ~0%.
The chemical formula of charcoal shows that in terms of carbon content it is close to stone, but in addition it has only a small amount of elements unnecessary for combustion (sulfur and nitrogen).
Activated carbon
Activated carbon is a type of carbon with a high specific pore surface area, which makes it even more absorbent than wood. The raw materials used for its production are charcoal and coal, as well as coconut shells. The starting material is subjected to an activation process. Its essence is to open clogged pores using high temperature, electrolyte solutions or water vapor.
During the activation process, only the structure of the substance changes, therefore the chemical formula of activated carbon is identical to the composition of the raw materials from which it was made. The moisture content of activated carbon depends on the specific pore surface area and is usually less than 12%.
fb.ru