The phenomenon of pyrolysis always accompanies the combustion of solid fuels in heating furnaces and boilers. The scale of the process depends on two factors - the combustion mode and the design of the home thermal power plant. We propose to consider in detail the pyrolysis of wood or coal, options for its use in industrial and domestic conditions. The goal is to dispel the myths invented by sellers and handicraft manufacturers of expensive “pyrolysis” equipment intended for heating private homes.
What is pyrolysis - description of the process
Theoretically, you can burn any substance that includes compounds of carbon and hydrogen, for example:
- coal;
- natural gas (methane, propane, etc.);
- biomass – fresh, dry;
- wood products, cellulose, ordinary firewood;
- various types of plastics;
- rubber made from natural or artificial rubber;
- oil, its derivatives;
- other carbon-containing waste.
At the output you will receive a certain amount of thermal energy, depending on the initial moisture content of the burned mass. To describe the processes, we use the chemical formula:
Combustion is a rapid oxidation reaction. Under ideal conditions, each carbon atom combines with two oxygen particles, and 2 hydrogen atoms interact with 1 oxygen particle. As a result, harmless compounds are formed - carbon dioxide CO2 and water. The latter evaporates when heated, taking away part of the released heat.
Important point. In real conditions, not all hydrogen and carbon atoms find a pair due to the lack of oxygen molecules. Therefore, the composition of combustion products includes a small proportion of harmful flammable compounds - carbon monoxide (CO), free hydrogen (H2) and carbon in the form of soot.
Even in a fire, pyrolysis gases are released - they burn above the main flame, combining with free oxygen.
Pyrolysis is a decomposition reaction of a substance that occurs when heated and there is a lack of free oxygen. This principle is used in gas generating plants:
- Fuel (in particular, wood) is placed inside a closed metal vessel - a reactor.
- The container is heated from the outside to 500...900 degrees, and a dosed amount of air is supplied through special holes - tuyeres.
- Under the influence of high temperature, the substance decomposes into 3 main components - carbon monoxide (CO), hydrogen (H2) and solid or liquid carbon residue. At the same time, a small amount of carbon dioxide and water vapor is formed.
- The volatile products constitute pyrolysis gas, a flammable mixture of hydrogen and carbon monoxide that leaves the container through a separate pipeline. The separated gaseous fuel is purified, cooled, and then pumped into the tank.
Diagram of the simplest gas generator installation with a water seal
Reference. Under production conditions, the resulting synthesis gas is sent to heat the same gas generator tank.
Combustion and pyrolysis are 2 different processes that can occur simultaneously. Example: during intensive burning of wood, a small volume of carbon monoxide is formed in the boiler furnace, while harmless CO2 is much larger. And vice versa, in smoldering mode, firewood releases a lot of hydrogen and carbon dioxide, some of which manages to turn into CO2 and oxidize. That is, it all depends on the amount of oxygen involved in the reaction.
Ecology DIRECTORY
The process of wood decomposition under the influence of heating in an oxygen-free gas atmosphere is carried out in a closed vessel (retort), heated through the walls. The retort has a pipe for removing the resulting vapor gases into a condensation device with a device for separating liquid from gas.[...]
Wood pyrolysis is carried out in closed vessels (retorts) of various designs when heated, by definition, without air access. At 120-150°C, water is removed, at 250-270°C, cellulose partially decomposes, and when the temperature rises to 450°C, decomposition of other wood substances is observed with rapid release of heat. At 450-550°C, the resulting coal is calcined and the remaining volatile substances are removed. When decomposing pine, spruce, birch and beech wood for 8 hours and at a final temperature of 400°C, about 32-38% of coal, 15-20 of gases and 45-50% of liquid are obtained. The latter is a solution of wood decomposition products containing,%: 6-12 acids, 3-5 alcohols and 5-7 resins. Resins are used for preserving wood, making roofing felt and other materials. Gases are sent to heat dry distillation retorts. [...]
The process of wood decomposition in industrial devices is divided into pre-drying of wood, pas:>-genetic decomposition and cooling of coal.[...]
The thermal decomposition of wood in the gas generator shaft occurs under different conditions compared, for example, with the process taking place in a retort with external heating. During gasification, dry distillation of wood occurs in a stream of hot steam gases that continuously penetrate a layer of wood chips. The presence of a relatively large amount of non-condensable gas promotes the evaporation of liquid products formed from wood. For this reason, the process of pyrolysis in the gas generator shaft can be identified with the decomposition of wood under vacuum. It is known that during the pyrolysis of wood under vacuum, the specific yield of liquid products characteristic of the conventional retort process increases, and, in addition, a number of such products are obtained from wood, for example carbohydrates, the presence of which is in the condensates formed during conventional dry distillation at atmospheric pressure in retorts with external heating, not observed. When wood decomposes in the gas generator shaft, the gas and the liquid and vapor products contained in it are continuously and gradually cooled. Therefore, almost no secondary processes, i.e., thermal decomposition of already formed products, occur. Relatively thermally unstable products remain in the gas almost without decomposition.[...]
Thermal decomposition of wood is a very complex chemical process.[...]
When wood is treated with liquid ammonia, some lignin is removed. Acetyl groups are converted into acetamide. Glucuronoxylanes were isolated from wood treated with ammonia, followed by extraction with water, in yields ranging from 2 to 10%. The study of these polysaccharides showed that they are not identical to the original xylan and are, apparently, products of its partial decomposition.[...]
Thus, heating wood during the period of drying and charring, before the onset of an exothermic reaction, is a mainly physical process, and the pyrogenetic decomposition of wood is a chemical process. [...]
When wood is crushed, its surface increases, receiving heat from gases during internal heating or heat from the walls of retorts and steam gases - pyrolysis products during external heating. It is advisable to saw firewood into short sections (dies, splints), which sharply increases the end surface through which pyrolysis products come out of hardwood wood, shortens the exit paths, speeds up the exit time, protecting pyrolysis products from re-decomposition. Firewood cut into short pieces is a bulk material, and all operations during its processing are easier to mechanize.[...]
If dried wood is placed in a closed steel vessel in an oven with a temperature of 700-800°, then rapid decomposition of the wood will begin, releasing a large amount of gas with a high calorific value (about 4000-4500 cal/m3). In terms of caloric content, such gas meets the requirements of gas for domestic purposes. In the 19th century, when there was no electricity, such gas from wood and coal was used for lighting. Hence, the name of the gas has been preserved to this day - luminous. Currently, this gas is more often called artificial household gas in contrast to natural gas. There is no high-temperature pyrolysis in the USSR, but its organization using wood waste by pyro-genetic means would be advisable if there is a need for household gas in areas rich in wood, but far from the places of extraction of fossil fuels, natural and liquid gas. Such gas is valued in the same way as power gas for internal combustion engines. Pyrolysis at high temperatures can be easily combined with the production of activated carbon, which should find wide application in agriculture.[...]
High humidity of wood undergoing pyrogenetic decomposition significantly reduces the efficiency of the process. In factories, when processing raw firewood in batch apparatuses, the first distillate runs containing a small amount. amounts of acids (up to 2-3%), are collected separately and are not allowed into processing or are released into the air in the form of vapors. It is very difficult to completely separate hygroscopic moisture during the process of dry re-heating of firewood in a gaseous environment due to uneven heating. Even in small pieces (chips), when the outer layers are re-charred, the drying process continues inside them and moisture inevitably enters the distillate.[...]
Exoenzymes secreted by the fungus into the wood are of primary importance in the decomposition of wood. The products of enzymatic breakdown are available to fungi and are a source of nutrition and energy for them. Endoenzymes are not released into the substrate. Remaining inside living cells, they ensure their internal metabolic processes. They enter the substrate as decomposition products of the fungal cell itself.[...]
The products of thermal decomposition of wood - gaseous, liquid and solid - consist of three main elements: carbon, hydrogen and oxygen. [...]
The gum was extracted from larch wood by hot water extraction. After evaporating the solution, the dry gum was subjected to thermal decomposition by heating for two hours at a temperature of 280-320°. [...]
In the processes of dry distillation of wood, peat or coal, the resulting gases and vapors are cooled, resulting in the formation of condensate enriched with decomposition products (gas water, tar water, etc.). Characteristic of these waters is the presence of an alkaline (during the processing of coal) or acidic (during the processing of wood and peat) reaction, as well as a different content of phenols and related compounds. The same applies to washing wastewater generated during the extraction from condensate and further processing of phenols, fatty acids, nitrogen and sulfur compounds. [...]
In practice, wood that goes into drying is very heterogeneous both in size of individual pieces and in quality. In the total mass of firewood entering the drying chamber, you can find thin and split wood, thick unsplit wood, healthy wood, and wood damaged by rot. Such heterogeneity creates great difficulties for establishing the correct operating mode of drying chambers and thermal decomposition of wood.[...]
The heat of reactions of thermal decomposition of wood can be determined by calculation, if only the percentage yield of the resulting products and thermochemical data on the heat capacity and heat of combustion of each of them are known. [...]
The highest thermal effect of wood decomposition reactions is the amount of heat released during the decomposition of wood, attributed to the liquid state of pyrolysis products, taking into account the heat that must be expended to overcome the external work of the released vapor-gas products. [...]
The results of experiments on the thermal decomposition of lignin containing 14% methoxyl groups, as well as hydrolytic lignin isolated by the hydrochloric acid method from pine, spruce and aspen wood, are given in Table. 18 .[ …]
Thermal constants of wood decomposition products are given in table. 22.[...]
The profitability of the process of wood pyrolysis in a stream of organic solvent vapors is determined by the losses of the latter during their conversion into steam. Typically, high-boiling solvents are steam distilled to lower the distillation temperature and reduce losses from cracking and tarring. It turned out to be advisable to immerse the distilled wood completely in a liquid organic coolant medium and distill it together with hygroscopic and then reaction water resulting from the thermal decomposition of wood. [...]
During the process of hydrolysis, wood polysaccharides are converted into corresponding monosaccharides, which dissolve in hot dilute acid. To protect these monosaccharides from decomposition at high temperatures, the hydrolysate containing them is continuously removed through filter 4 throughout cooking and quickly cooled in evaporator 6. Since, according to the process conditions, the hydrolyzed plant raw material must be filled with liquid at all times in the hydrolyser, the specified level e[...]
It occurs during the pyrogenetic decomposition of wood in the composition of phenols in the volatile part of pyrolysis gases (Lipina).[...]
The relationships of fungi in the process of wood decomposition are determined by the fact that a fungus capable of decomposing healthy wood prepares the substrate for the next species. It must be remembered that in the process of depletion of nutrients, the fungus that settled first becomes less viable, while the one for which partially decomposed wood is the optimal environment acquires the most favorable conditions for development and it relatively easily displaces its predecessor. According to Ripach e-k, such a pair is, for example, the bordered polypore (Fomitopsis pinicola) and the odorous polypore (Osmoporus odoratus). The first mushroom settles on healthy stumps, sometimes even on living trees. The odorous tinder fungus destroys wood much more slowly, but, as experiments show, after a month of development of the bordered tinder fungus, the activity of the odorous tinder fungus on prepared wood increases significantly. However, it should be taken into account that a change in temperature-psychrometric conditions changes the metabolism of fungi, and therefore the possible sequence of their development.[...]
Research on the pyrogenetic decomposition of cellulose shows;:” that the decomposition process proceeds in the same way as wood, i.e. with the formation of a large number of different decomposition products. [...]
The amount of heat released during the decomposition of wood is not constant and depends on the quality and type of wood, the rate of temperature increase during the exothermic reaction, the total pressure in the apparatus, the method of heating the wood and the type of apparatus. Therefore, the thermal effect of wood pyrolysis reactions varies somewhat in each individual case. Thus, rapid heating to a higher temperature promotes the flow of secondary reactions during the decomposition of cellulose, lignin and other constituent substances of wood. Thanks to this, the overall process of wood decomposition will proceed with a smaller thermal effect, which can be explained in accordance with the well-known rule indicating that exothermic reactions preferentially occur at low temperatures, and endothermic ones at high temperatures. If the pyrolysis process proceeds with the release of heat, the calorific value of wood will be greater than the calorific value of the products of thermal decomposition, and if heat would have to be expended for decomposition, then the calorific value of wood would be less than the calorific value of the decomposition products. [...]
In cooking processes and during hydrolysis of wood, either the aromatic part (delignification during pulping) or the carbohydrate part (conversion of polysaccharides into monosaccharides in hydrolysis production) is subject to destruction. When wood is heated without air access, decomposition (thermal destruction) of all wood components occurs. This process is called dry distillation or pyrolysis of wood.[...]
In both cases, as a result of heating, vapor and gaseous decomposition products (vapor-gas mixture) are released from the wood, and charcoal remains. If they want to turn wood into gaseous fuel, then charcoal is not obtained, but due to its interaction at high temperatures with atmospheric oxygen and water vapor, combustible gas and heat are obtained for the pyrolysis of wood. This process is called gasification, and the resulting vapor-gas mixture is called raw generator gas.[...]
Methyl alcohol is obtained from the pyrogenetic decomposition of wood as a product of the distillation of tar water. Other distillation products are wood chemical solvents (A, AMA, MAC, etc.), which also include methyl alcohol (E. A. Peregud). The methyl alcohol obtained by this method is contaminated with its accompanying products, as a result of which it is not suitable for the synthesis of pharmaceuticals, for the production of formaldehyde, etc. For these purposes, synthetic methyl alcohol is used, obtained from carbon monoxide and hydrogen at high pressure in the presence of a catalyst. [...]
Klason and his co-workers 1 studied the pyrogenetic decomposition of cellulose from cotton, pine, spruce and birch. Pulp from pine and spruce. birch was isolated from wood using the sulfite method. The laboratory apparatus was filled with 1 kg of cellulose in the form of briquettes with a specific gravity of 1.2.[...]
As already mentioned, the amount of heat released during the decomposition of wood of the same composition is not constant, but depends mainly on the pressure at which the pyrolysis process occurs and on the rate of temperature increase, especially during the period of the exothermic reaction. [... ]
It should be emphasized that the final product of the combustion of all types of fuel, the decomposition of all types of organic matter, the oxidation of CO and a number of other processes occurring with the participation of carbon and its compounds is carbon dioxide CO2. CO2 differs from other gaseous man-made emissions in that under natural conditions it is produced in huge quantities and its circulation in the biosphere is one of the fundamental processes of mass and energy exchange in nature and the maintenance of life on Earth. Carbon dioxide itself is not a toxicant, but in the 20th century. its average planetary concentration in the air began to increase annually by 0.8-1.5 mg/kg. This is caused by the combustion of fossil fuels (5 ■ 109 t/year in terms of carbon), the use of agricultural raw materials and wood (5 • 109 t/year), which is equivalent to the annual release into the atmosphere (30-42) ■ 109 t of CO2. [... ]
Currently, in practice, the extraction of resins and acids from the steam gases of wood charring is carried out by irrigation with a neutral solution of wood vinegar powder. In industrial conditions, this method of charring wood consists of the following stages: drying the wood, decomposing it and cooling the coal 2.[...]
Cellulose-degrading and lignin-degrading fungi consume cellulose or lignin, due to which the wood acquires a reddish-brown or light color. Lightening of wood during decomposition by lignin-destroying fungi is caused by oxidative enzymes that fungi release into the wood. The darkening of wood caused by cellulose-degrading fungi depends on humic substances. Some wood-destroying fungi also produce pigments that color the wood in different colors. Similarly, root sponge in the first stage of decomposition turns wood into shades of purple. In wood-destroying fungi, changes in the color of hyphae are also observed. The same root sponge forms colorless hyphae in tracheids. In the medullary rays, the hyphae are brown and cause a brownish-violet coloration.[...]
The rate of temperature increase during the exothermic reaction has a decisive influence on the processes of wood decomposition and on the amount and composition of the forming vapor-gas mixture. [...]
Depending on the type of forest, tree species and other conditions, the composition of species and genera of fungi at different stages of decomposition may vary somewhat. For example, when pine and spruce wood decompose, different types of penicillium develop. In addition, more species of this genus, etc., take part in the process of decomposition of spruce wood [...]
The progress and results of pyrolysis largely depend on the environment in which the heated wood is located. The usual medium most often encountered in practice is a gas, or, more precisely, steam-gas medium. In the retort, wood undergoes pyrogenic decomposition in a weak current due to the natural convection of its decomposition products. In circulation retorts (Kozlov furnace, retort of the Amzinsky plant), the medium is non-condensable gases - products of wood decomposition mixed with water vapor and a small amount of organic substances. The latter are present in the gas due to their incomplete extraction in the circulation-condensation system. In gas generators and the TsKTI furnace, the medium is generator gas, which contains superheated water vapor, partially remaining from steam-air blast, and in the upper layers of the shaft - pyrolysis products of wood from the lower layers. Everything that is said above about operating factors applies to the listed types of vapor-gas environment.[...]
However, it is not the higher thermal effect that is of practical importance, but the lower one, since the resulting vaporous products of wood decomposition are not removed from the apparatus in a liquid state. and in vapor form.[...]
An essential feature of woodchip gasification is the low temperature of the gas at the outlet of the gas generator, the increased speed of the process of drying and decomposition of wood and the rapid removal of vapor and liquid products from the reaction sphere, which ultimately leads to increased yields of liquid products of wood pyrolysis. [...]
In Russian literature, pyrogenic decomposition of wood is usually called dry distillation of wood. Sometimes the word carbonization is used, from the German schwelen - to drive resin. You read the word pyrogenetic decomposition and replace it with the shorter term pyrolysis.[...]
Fresh stumps, while in the soil, undergo gradual destruction. The sapwood part of the stump is destroyed very quickly. It acquires pronounced traces of decomposition already in the first year after felling the tree. Its color becomes dark, the wood becomes damp, acquires viscosity, and is difficult to chip.[...]
Hemicelluloses from other hardwood species have been studied to a much lesser extent; Usually only the general structure of one of the polysaccharides is known.[...]
Using this method, the authors sampled the lignins, compared them with the corresponding native Brauns lignans, and found that they were not significantly different. Subsequently, other microorganisms were used to decompose wood /31/. The long time required for the decomposition of the saccharide part by microorganisms (13-15 months), as well as the possibility of changes in lignin during their action, were probably the reason why this method of lignin isolation has not become widespread. [...]
When heated to 250-300 Oe, it produces rosin oils, which contain a very large number of products. Such decomposition products of rosin are contained in resin obtained from the pyrogenetic processing of coniferous wood.[...]
The process of decomposition of wood (lignin) involves slightly different microorganisms than those listed above. In the decomposition of lignin, the role of fungi appears to be more important.[...]
In this way, flavonoid compounds can be isolated from extracts of fruits, leaves, wood and roots. The only obstacle may be the tendency of glycosides to hydrolysis on an acidic column. This type of excipients can be used to decompose metal salts of flavonoids, separating free phenols from metal ions by washing with alcohol. [...]
The increase in yields when using water vapor is explained by the fact that the removal of valuable products from the reaction space is accelerated and the development of secondary decomposition reactions is delayed. In addition, when water vapor comes into contact with the capillary system of wood on its surface layers, steam condensation is possible, which creates conditions for thermal decomposition in an acidic aqueous environment. In this case, decomposition reactions occur primarily in the layers of the cell wall, which are located on the inner sides of the cell cavities and consist mainly of non-heat-resistant hemicelluloses, which easily eliminate acetyl groups and part of the methoxyls associated with them, forming acetic acid and methyl alcohol, respectively. [...]
The main pyrogenetic process was chosen to produce charcoal, which is a more scarce and needed product than wood generator gas. To obtain the largest range of pyrolysis products formed at low and high temperatures, the decomposition process is carried out in two stages. First, the wood is subjected to preliminary pyrolysis in a liquid coolant (diesel fuel) at a temperature of 275° and the bulk of acids, low-boiling products included in the so-called wood alcohol, and resins are obtained. The brown wood formed as a result of pre-pyrolysis (see page 37) is subjected to secondary pyrolysis at a temperature of 600-700 ° with a solid coolant (charcoal) and an illuminating gas and liquid are obtained containing settling resin with a high yield of low-boiling phenols, additional acids and wood coal. The latter is characterized by a low volatile content and increased activity.[...]
In addition, a rapid increase in temperature during an exothermic reaction promotes the formation of CO instead of CO2 and H0, since the process of formation of the latter, as is known, proceeds with a large release of heat. An increase in the yield of CO instead of C02 and H20 causes a decrease in the yield of coal by weight and a decrease in the percentage of carbon per unit volume of coal. Therefore, the process of charring wood will be rational if it proceeds under conditions that would exclude the possibility of increasing the temperature during the exothermic reaction of wood decomposition. The creation of charcoal conditions, as will be indicated below, depends on the method of burning wood, the type of charcoal burning apparatus and the moisture content of the wood. [...]
Leaching of trace elements and their inclusion in migration processes occurs not only as a result of the influence of abiogenic factors on rocks and the products of their mechanical destruction. Living organisms also play an active part in this. Some of them, primarily woody plants, extract ore elements, including heavy metals, from the depths using their root systems. The subsequent decomposition of leaf litter and dead wood leads to the enrichment of the surface layer of soil with these elements. Consequently, we can talk about the functioning of a kind of geochemical, or rather biogeochemical pump (V.M. Goldshmidt), due to which geochemical anomalies are often formed on the surface.[...]
Effect of high humidity
A high moisture content in the starting material has an equally detrimental effect on combustion and pyrolysis reactions. Let's look at the processes using wood burning as an example:
- During combustion, the energy released is spent on evaporating the water contained in the wood. The amount of heat at the output is significantly reduced, and the fuel is burned in vain.
- Moisture greatly slows down the thermal decomposition of the substance. Part of the heat expended on heating is taken away by evaporating water, and the required temperature (minimum 500 °C) is not achieved. Pyrolysis of wood containing more than 50% moisture is practically impossible.
The best humidity indicator for fruitful combustion or decomposition of wood in a gas generator is 8...15%. At home, it is impossible to achieve such indicators; long-term drying of firewood under a canopy allows you to achieve 20-25% moisture content.
Reference. When producing fuel pellets and briquettes at the plant, sawdust is dried to 8-10%. The maximum moisture content of finished granules is 15%.
Wet firewood burns poorly and smokes heavily because the heating releases water vapor and soot.
Pyrolysis of sawdust
Pyrolysis of sawdust is the most profitable way to dispose of wood waste. Thanks to this technology, waste from the wood processing industry does not need to be taken to a landfill for disposal, but can be used to generate heat and electricity.
In recent years, this use of wood waste has begun to be seen as an excellent alternative to traditional fuels. All this is directly related to the fact that sawdust as a fuel has a number of advantages:
- they belong to renewable sources of thermal energy
- are completely CO2 neutral
- There is practically no sulfur in sawdust
- it is possible to burn wet waste (containing up to 55 - 60% moisture)
- The corrosiveness of flue gases is quite low
- low, compared to fossil fuels, price of raw materials
Using wood waste as fuel is not only much less harmful to the environment, but also saves money. This way of saving non-renewable natural resources can allow Russia to get closer to more developed countries in terms of such an indicator as the specific energy intensity of industrial production, which makes it extremely attractive. And all this leads to the fact that sawdust pyrolysis technologies have been constantly developing and improving in recent years.
What is thermal decomposition used for?
The scope of application of pyrolytic processes is quite wide:
- Production of propylene and ethylene for the chemical industry by processing liquid hydrocarbon feedstock (oil).
- Preparation of charcoal by the method of oxygen-free decomposition of wood waste.
- The same technological process, but with a limited air supply, makes it possible to produce flammable synthesis gas from wood - a mixture of methane, hydrogen, carbon monoxide and neutral nitrogen.
- Pyrolysis of coal – brown and hard – is a whole area of processing. The resulting compounds are synthetic gasoline, coke, ammonia, and coal tar. Toluene, benzene, naphthalene and various phenols used in the chemical industry are extracted from the latter.
- New developments - commercial recycling of municipal solid waste, car tires, plastics, organics.
Note. Listed here are the most well-known uses of pyrolytic reactions. In reality, there are many more use cases. Wikipedia states that pyrolysis processes have not been fully studied; many projects are at the development stage.
For thermal decomposition in industry, pyrolysis furnaces and various reactors are used. The diagram above shows a gas generator plant that processes wood waste and sawdust into gaseous fuel. The main role here is played by a direct dry distillation reactor, where the prepared raw materials are processed into synthesis gas by slow combustion.
An important nuance. Before loading into a pyrolysis oven or gas generator, wood is always crushed and dried to a moisture content of 10% or less.
In industrial chemistry, fast pyrolysis technology is also used, when the reactor is heated to a temperature of 700...900 °C over a short period of time. The goal is to increase equipment productivity and speed up processing.
Wood pyrolysis products
Currently, hardwood is usually used to carry out the process of wood pyrolysis, but sometimes (mainly during complex processing of raw materials) coniferous wood is also used. Modern pyrolysis technologies make it possible to obtain from birch wood:
- charcoal - 24-25% charcoal,
- liquid waste (so-called slurry) - 50-55%
- gaseous products - 22-23%
The larger the size of the pieces of wood taken for pyrolysis, the larger the solid residue will be. The charcoal obtained as a result of pyrolysis, after the sorting procedure according to the size of the pieces, is sent directly to the consumer or for processing.
When processing the liquid obtained as a result of pyrolysis, wood resin (which is approximately 7-10%) settles and at the same time numerous transformations of the components occur. A wide range of valuable products can be distinguished from resin. As a rule, acetic acid is isolated from the liquid. It is usually extracted from the liquid by extraction, and then, through rectification and thorough chemical purification, it is processed into a food product ready for sale.
Use in everyday life
At the household level, pyrolysis helps solve the following problems:
- cleaning the oven or fryer from sticky fat deposits that cannot be removed mechanically;
- production of charcoal;
- heating a private house using a pyrolysis solid fuel boiler.
The best method to clean a frying pan is to place it in the oven, set the temperature to 200...250 °C and leave for half an hour. Without access to oxygen, deposits will be destroyed, only ash will remain, and the pyrolysis gases will be taken away by the kitchen hood.
Reference. There are oven models with a built-in pyrolytic cleaning function. At the end of the “roasting”, all that remains is to wipe the internal surfaces and throw away the resulting ash.
Charcoal is used for barbecuing, blacksmithing and more exotic purposes - refueling a car gas generator (read how it works in a separate article). The method of production is burning wood waste inside a closed container, that is, slow pyrolysis.
The feasibility of purchasing and operating pyrolysis boilers is a rather controversial issue. What is alarming: even the sellers presenting heating gas-generating equipment at the famous Aquatherm exhibition are unable to clearly explain what pyrolysis is. If you don't believe me, watch the video:
We propose to analyze in detail the problems associated with pyrolysis wood heat generators.
What happens to the material?
During operations, the structure of the material changes at the molecular level. Fibers and bonds are broken down by temperature and pressure. As a result, the surface becomes moisture-resistant, resistant to deformation, rotting, infestation by parasites and less porous. Thermal wood tolerates serious temperature changes, humidity surges and no longer deforms under heavy rains, even without additional protective coatings. Treated lumber does not rot, does not harbor mold or insects, which means it can last at least 20 times longer than regular wood.
The new aesthetic characteristics of the material deserve special attention. During the process of heat strengthening, it changes color - it acquires a shade characteristic only of expensive varieties. So, from the simplest material one can get something more valuable in appearance - for example, similar to larch.
Heat treatment, depending on the temperature regime, is divided into several classes:
- 1st – processing of materials with low rates and a light degree of toning at temperatures up to 190 °C;
- 2nd – the material is painted dark and gains strength during drying at a temperature of 200 °C;
- 3rd – processing at 240 °C allows you to obtain high-quality lumber that is resistant to external factors, hard and dense, with an even dark shade and a noble texture.
Myths about pyrolysis TT boilers
The main design difference between a gas generator heater and a traditional direct combustion boiler is 2 chambers instead of one. A ceramic nozzle is installed between both fireboxes, and the air is forced into the air by a fan. The metal walls of the pyrolysis unit are protected by a lining of refractory bricks. How does he work:
- Firewood or coal is placed in the upper (primary) chamber and set on fire.
- Automation starts the boost fan.
- When the temperature in the firebox rises to 500 degrees, the release of pyrolysis gases begins.
- Entrained by the general flow of combustion products, these volatile compounds enter the lower secondary chamber, where they are burned in the presence of oxygen (supposedly).
Cross-section of a gas generator heater
In reality, the resulting synthesis gas begins to burn in the primary firebox, since the fan supplies excess air. Only a torch of flame is directed into the second chamber... and that’s all. Then the combustion products move through the flame tubes of the heat exchanger, heat the coolant and fly away into the chimney.
Addition. There is another design of heaters - without a fan, the secondary chamber is located at the top. From the point of view of pyrolysis, the concept is unworkable; the unit functions like a regular wood-burning hot water boiler, although it costs twice as much as its classic counterparts.
Supporters of pyrolysis heat generators (this includes manufacturers of this equipment, sellers and home craftsmen) attribute the following advantages to their TT boilers:
- the fuel is burned completely, the residue in the ash pan is practically zero;
- burning duration – 10 hours or more;
- low volume of harmful emissions into the atmosphere;
- high efficiency due to efficiency of 86...90% (manufacturers’ indicators) compared to traditional boilers with an efficiency of 75%.
Let's try to understand the veracity of these statements. Point one: if the firebox is loaded with dry wood (these are required according to the heater operating instructions), then after combustion there will be fine ash left. The air flow created by the fan and accelerated in the nozzle will simply blow the light residue into the chimney.
Due to the forced injection of gases from the side of the furnace, only a large fraction of ash remains in the secondary chamber
The result is an almost empty ash pan, the illusion of complete combustion. If you put dry wood into a classic turbocharged TT boiler, you will get a similar residue - a little ash at the bottom. That is, the completeness of combustion depends on the quality of the fuel, and not on the design of the heat generator.
Comment. Stacking raw firewood with a humidity of over 50% will give a negative result in any boiler. There is no point in considering such options.
Let us briefly answer the remaining statements:
- The burning time of 10-12 hours corresponds to reality. Another thing is that this indicator is achieved due to the size of the fuel chamber (100 liters or more), where a lot of firewood is placed. Pyrolysis has absolutely nothing to do with it.
- The assurances about the environmental friendliness of the boiler are true. The fan pumps air in excess, producing very little toxic gases. In standby mode, oxygen does not enter the firebox, the wood smolders slowly and the amount of harmful emissions increases.
- Boiler efficiency of 90% is fairy tales. In active combustion mode, the principle of operation of the boiler is similar to turbocharged versions of traditional units, whose efficiency does not exceed 75%. When the fan is turned off, the flame dies out and the smoldering coals emit little heat.
Conclusion. Purchasing a gas generator model of a solid fuel boiler is a very dubious undertaking. The unit is three times more expensive than conventional versions and twice as heavy due to the lining. Homemade heat generators, as a rule, are more reliable and cheaper than factory ones, but are too bulky. In terms of efficiency and other characteristics, they do not outperform classic TT boilers with a turbine or chain draft regulator.
Our opinion will be confirmed by a well-known expert practitioner in his video:
Primary products of wood pyrolysis
Gaseous, liquid and solid products of wood pyrolysis consist, like the original wood, of three main elements - carbon, hydrogen and oxygen; they also contain a small amount of nitrogen-containing substances.
Gases.
The composition of gases formed during the pyrolysis of wood depends little on the type of wood.
Their composition when wood is recharred at 400 °C (in volume %) is given below.
Components of gases. C02 CO CH4 C2H4 H2
Birch. 49.0 28.4 18.2 1.4 3.0
Pine. 49.5 28.5 18.0 1.0- 3.0
Spruce. 48.0 28.0 19.0 1.0 4.0
The pyrolysis of 1 m3 of wood produces 75–90 m3 of non-condensable gases.
The lower (useful) heat of combustion of 1 m3 of non-condensable gases, kJ/m3, can be calculated using the formula
Q„ = 127.5 CO + 108.1 H2 + 358.8 CH4 + 604.4 C2H4, where CO, H2, CH4, C2H4 are the volumetric content of these gases in the mixture, %.
Liquid products.
The condensate obtained by cooling the vapor-gas mixture formed during the pyrolysis of wood is called liquid or raw liquid.
3.1. Composition of the liquid obtained from the pyrolysis of wood in a vertical batch retort, %
Methyl alcohol, .-;.u.
Water (varies)
The raw liquid has a density of 1.02–1.03 g/cm3. It contains a variety of organic substances, both soluble and insoluble in water. Some of the water-insoluble substances can dissolve in the liquid, while others are suspended in it, in the form of tiny suspended droplets. As they settle, they separate from the water layer, forming a settling resin that collects at the bottom of the settling tank. In addition, when some wood species, in particular birch and aspen, are over-charred, a small layer of light oils is formed that float to the surface of the liquid and are similar in composition to tar; these oils are formed mainly due to the decomposition of the bark. In addition to settling resin, the liquid also contains soluble resin, which is separated only when the liquid is distilled.
The composition of the slurry (Table 3.1) depends on the type of wood being recharred, its moisture content and the conditions of the process. It contains: formic, acetic, propionic, butyric, valeric acids, etc.; methyl alcohol, propyl alcohol, allylic alcohol, etc.; ketones acetone, methyl ketone, methyl propyl ketone, methyl butyl ketone, etc.; aldehydes formaldehyde, acetaldehyde, furfural, etc.; methyl esters of acetic and other acids; phenols, phenol ethers and many other compounds.
The better the wood is dried, the lower the yield of liquid, but the correspondingly greater the concentration of useful components in it. When recharring birch wood, dried to a moisture content of 8-10%, the yield of sludge is 280-295 kg/m3; from air-dried wood, 350-380 kg/m3 of slurry is obtained, etc. From 1 m3 of birch wood, more slurry is obtained than from aspen , since birch wood has a higher density.
The decomposition process of dry and wet wood occurs differently. Dry wood, with a moisture content of less than 10%, releases more heat per unit time during decomposition than wet wood; The exothermic reaction begins faster and proceeds more violently, the process accelerates, and the coal yield decreases. During the decomposition of damp wood, the process seems to self-regulate: the temperature decreases due to the high heat consumption for moisture evaporation, the exothermic reaction is extended and the rate of charring decreases, as a result of which the yield of acids and coal increases slightly. It would seem that recharring raw wood is more appropriate. However, this is not so: the use of retorts as drying devices is irrational, and the processing of low-concentrated liquid requires an increase in the size of the equipment and increased heat costs.
When wood is artificially dried, moisture evaporates from its surface, and at the same time moisture moves from the wetter, inner layers of wood to the less humid, outer layers. Both of these processes accelerate with increasing temperature, but the second of them proceeds more slowly, which leads to cracking of wood and a decrease in the physical and mechanical properties of the coal obtained from it. To avoid this, you should limit the drying temperature and use a partially humidified coolant. An important factor in drying is also the circulation of the coolant in the drying device, which is necessary to supply heat to the material being dried and remove evaporated moisture.
A significant increase in the drying temperature is allowed when drying lumps in a continuous dryer with parallel flows of wood and coolant. In this case, the wettest wood comes into contact with a coolant heated to 300 °C, and partially dried wood comes into contact with a significantly cooled coolant, which protects it from burning.
The size of the wood pieces has a significant influence on the yield of products, in particular acetic acid. Thus, 8% more acetic acid is obtained from lumps than from meter-long pieces.
To speed up the pyrolysis process, obtain a sufficiently concentrated liquid and reduce fuel costs for its processing, you should use lumps dried to a moisture content of 10-15%.
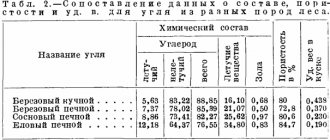
Solid products (charcoal).
Under the same conditions of recharring wood of different species, coal has almost the same composition. With increasing temperature of recoaling, the yield of coal decreases, but at the same time its quality increases (Table 3.2); The yield of coal also decreases slightly with the acceleration of recoaling.
The coal should not be over-burnt or under-burned. Burnt coal is produced by the action of free acid